Sex-related differences in structure, function and connectivity of central arousal and salience networks involving brainstem nuclei are involved in IBS symptom generation
Overall Goal
To identify sex-related differences in functional and structural aspects of dorsal brainstem regions and related neural networks of IBS subjects, determine their relationship to specific gut microbiome features, as well as clinical and physiological parameters. Sex-related changes in the brainstem microstructure and corresponding alterations in brain cortical networks have never been studied in IBS patients.
Background
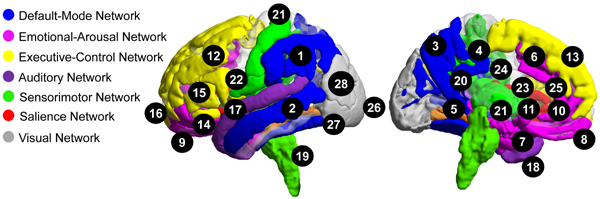
Previous research and our preliminary data support the hypotheses that sex as a biologic variable (SABV) influences IBS-related alterations in the structure, functional and organization of salience, sensorimotor, and emotional-arousal regions in the brain, as well as the reactivity of salience and emotional-arousal regions. As shown in the figure, female IBS compared to male IBS demonstrated moderate to large reductions in grey matter measures in default mode, emotional arousal, executive control, salience, primary sensorimotor regions (including posterior insula, basal ganglia, thalamus, and brainstem), and multimodal (auditory, visual, and visuospatial, convergence zones) regions. This data suggests that sex-specific structural alterations may underlie sex-specific functional alterations reported by IBS patients.
Sex-differences in resting state functional magnetic resonance imaging (fMRI) studies demonstrate sex differences in sensorimotor, salience and emotional-arousal alterations in IBS. Female patients show greater lower frequency power in the sensorimotor cortex and higher frequency power in the insula and amygdala compared to that in male patients and female healthy controls. Furthermore, sex influences the nature of insula intrinsic connectivity changes in IBS. The findings suggest sex differences in internally-directed resources in response to stressful and salient events. Finally, variations in the topographical organization of connectivity between the sensorimotor system and regions providing emotional, salience, and default mode networks inputs may result in biased and unreliable processing of sensory information.
General Hypotheses
- IBS subjects show sex-related structural and functional alterations in specific brainstem regions that influence brain networks which play crucial roles in altered sensory processing and modulation, reflected in cardinal IBS symptoms.
- These brain alterations are associated with plasma and/or fecal levels of neuroactive gut microbial metabolites, including but not limited to short-chain fatty acids, and microbial metabolites related to estrogen, tryptophan and bile acids. We hypothesize some of the involved metabolites signal to the brain and pontine nuclei via vagal and/or spinal afferent pathways, while others can cross the blood-brain barrier and reach monoaminergic brainstem nuclei via the systemic circulation.
Specific Aims
Aim A. To quantify sex-related differences in brain function within disease- relevant networks and the pontine modulation of these networks at rest and during pain expectation in IBS patients.
This proposal applies advanced neuroimaging acquisition and advanced bioinformatics techniques to test the general hypothesis that functional and structural alterations occur in the brain and brainstem in IBS in a sex-specific manner.
Aim B. To accurately localize sex-related anatomical and microstructural differences within the brain and brainstem using advanced diffusion imaging, 7T imaging, and neuromelanin-specific imaging.
We will characterize alterations in the previously mentioned brain networks and their interconnectivity in male and premenstrual female IBS subjects, and identify the role of ascending noradrenergic and serotonergic influences from respective brainstem nuclei in these alterations.
Aim C. Correlate sex-related differences in brain function and structure with clinical features and gut microbiome characteristics.
We will aim to determine if these alterations are associated with serum and gut microbial parameters, as well as clinical and behavioral measures
People
Project Leads: Benjamin Ellingson, PhD (Co-Lead), Jennifer Labus, PhD (Co-Lead)

Benjamin Ellingson, PhD
Director, UCLA Brain Tumor Imaging Laboratory (BTIL); Co-Director, Center for Computer Vision and Imaging Biomarkers; Professor, Department of Radiology at David Geffen School of Medicine
As Director of the UCLA Brain Tumor Imaging Laboratory and Co-Director of the UCLA Center for Computer Vision and Imaging Biomarkers (CVIB) his research focuses on the development, testing, validation, and implementation of advanced MR and PET imaging biomarkers for brain pathology and response evaluation in clinical trials. He possess a broad background in biomedical engineering, image processing, MR and PET imaging physics, functional and molecular imaging, bioelectronics, medical instrumentation, and statistical parameter mapping. He has been co-author on more than 100 peer-reviewed original research articles relating to advanced neuroimaging and medical imaging physics. He has wide-ranging experience in designing and implementing multicenter neuroimaging trials. This includes trials in primary and metastatic brain cancers; neurotrauma including traumatic brain injury (TBI), traumatic spinal cord injury (SCI), and degenerative spinal disease; psychiatric diseases including schizophrenia; epilepsy, tuberous sclerosis complex, and other neurodegenerative diseases; and chronic pain syndromes including cervical spondylotic myelopathy, irritable bowel syndrome (IBS), chronic headaches, and urological chronic pelvic pain syndrome (UCPPS). He is also the principal investigator for the imaging core in numerous industry-funded therapeutic clinical trials in brain tumors, chronic pain, epilepsy, and schizophrenia.
In this proposal, he will be Co-Lead of Project 2 and will be responsible for the design and analysis of all brainstem and brain MRI experiments, including optimization of protocols for both 3T and 7T imaging. His laboratory will post-process anatomic and diffusion MR imaging data, and work closely with Neuroimaging and Bioinformatics Core to identify sex-related differences in the brain and brainstem within IBS patients and the association with clinical symptoms and gut microbial parameters.
Publications
http://www.ncbi.nlm.nih.gov/sites/myncbi/1t5OXTmr85Skz/bibliography/50293169/public/

Jennifer Labus, PhD
Director, Biostatistics and Bioinformatics Core, G. Oppenheimer Center for Neurobiology of Stress and Resilience; Adjunct Professor, Vatche and Tamar Manoukian Division of Digestive Diseases, David Geffen School of Medicine at UCLA
Dr. Labus is an Adjunct Professor in the Vatche and Tamar Manoukian Division of Digestive Diseases in the Department of Medicine at University of California, Los Angeles (UCLA). She is the Director of the Integrative Biostatistics and Bioinformatics Core in the G. Oppenheimer Center for Neurobiology of Stress at UCLA and the UCLA Microbiome Center.
Dr Labus is an applied statistician with expertise in biostatistics, bioinformatics, treatment-outcome research, pain neuroscience, multimodal brain imaging, microbiome, metabolomics, and multi-omics integrative analysis. Her current research focused is on determining biological markers of disease, including chronic pain, obesity and Alzheimer’s disease. Using state-or-the-art computational, biostatistical, and bioinformatics approaches, she assesses the interaction between various levels of biological data (e.g., microbiome, metabolomics, immune markers, multimodal brain imaging data) with clinical data. The overall goal of her systems-based approach is to identify and target the key regulators of multi-omics-biological disease-interaction networks in order to understand the underlying pathophysiological mechanisms and provide new targets for treatment.
Dr Labus has made seminal contributions to mapping neural networks underlying visceral pain and elucidating brain-gut –microbiome axis in humans. As a result, she was the recipient of the 2011 Master’s Award for Outstanding Achievement in Basic or Clinical Digestive Sciences, American Gastroenterology Association. Dr Labus has been the recipient of a K08 Career Development award, Effective connectivity of central response in irritable bowel disorder, from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). She has served as the primary investigator on two grants funded by the National Institute of Childhood Health and Human Development (NICHD): R01HD076756 Profiling vulvodynia subtypes based on neurobiological and behavioral endophenotypes and R21HD086737 Deriving novel biomarkers of localized provoked vulvodynia through metabolomics: A biological system-based approach. Labus is a co-investigator on several NIH funded grants, international research collaborations, and is actively involved in mentoring graduate students and postdoctoral fellows.
Publications
http://www.ncbi.nlm.nih.gov/sites/myncbi/1TAcC6itlmG/bibliography/44260598/public/
Studies
Conducted by Emeran Mayer, MD and Lin Chang, MD
Help answer questions related to sex differences in the structure and function of our brains and chronic abdominal pain. Participate in Brain Imaging Research at the G. Oppenheimer Center for Neurobiology of Stress and Resilience.
 You must:
You must:
- Have chronic abdominal pain with altered bowel habit for at least 6 months or have been diagnosed with irritable bowel syndrome (IBS)
- If female, not pregnant, breast feeding or on hormone-based birth control
- Be between the ages of 18-55
- If female, not pregnant, breast feeding or on hormone-based birth control
- Have no other significant medical or psychological history
Participation involves a screening visit, online questionnaires, an MRI and one sample each of blood, stool and saliva.
Earn up to $265 and get a digital image of your brain.
If interested, please call Cynthia at (310) 206-1719
Reference: SCORE P2
Conducted by Emeran Mayer, MD and Lin Chang, MD
Help answer questions related to sex differences in the structure and function of our brains and chronic abdominal pain. Participate in Brain Imaging Research at the G. Oppenheimer Center for Neurobiology of Stress and Resilience.
 You must:
You must:
- Be between the ages of 18-55
- If female, not pregnant, breast feeding or on hormone-based birth control
- Have no significant medical or psychological history
- Have no close family history of chronic pain syndromes
Participation involves a screening visit, online questionnaires, an MRI and one sample each of blood, stool and saliva.
Earn up to $265 and get a digital image of your brain.
If interested, please call Cynthia at (310) 206-1719
Reference: SCORE P2
