 Irritable bowel syndrome (IBS) and chronic constipation are a female-predominant disorders. There are significant sex-related differences in the clinical presentation of IBS suggesting sex as a biologic variable (SABV) plays an important role: IBS is more common in women than men, symptoms are more severe during the perimenstrual period and menopause, symptomatic women seek healthcare for IBS more often, and women with IBS exhibit higher levels of hypersensitivity to intestinal and non-intestinal stimuli along with reporting more extraintestinal comorbidities.
Irritable bowel syndrome (IBS) and chronic constipation are a female-predominant disorders. There are significant sex-related differences in the clinical presentation of IBS suggesting sex as a biologic variable (SABV) plays an important role: IBS is more common in women than men, symptoms are more severe during the perimenstrual period and menopause, symptomatic women seek healthcare for IBS more often, and women with IBS exhibit higher levels of hypersensitivity to intestinal and non-intestinal stimuli along with reporting more extraintestinal comorbidities.
These disorders are more prevalent in women than men and their pathophysiology is not completely understood. While there are some treatments available to treat symptoms, the majority of patients report lack of satisfaction with current treatments. The SCORE proposed studies will compare brain-gut microbiome (BGM) interactions in 1) women with IBS and healthy women during low estrogen states (perimenstrually, post-menopausal); 2) women and men with IBS and healthy women and men, and 3) premenopausal women with normal and slow transit constipation and healthy women. In addition, the proposed studies will study gut microbial and brain mechanisms underlying the effectiveness of cognitive behavioral therapy in women and men with IBS. Results obtained from the proposed studies are likely to lead to provide significant insights on the pathophysiology and treatment of these disorders.
IBS has a worldwide prevalence of 4-11% with higher rates in women. It is characterized by abdominal pain associated with diarrhea and/or constipation. IBS is currently defined by symptom-based Rome IV criteria as chronic or recurrent abdominal pain occurring on average, at least once a day per week in the previous three months associated with at least two of the following: pain with defecation, change in stool frequency, and change in stool appearance. There are three main IBS bowel habit subtypes that are based on the prevalence of abnormal stools: diarrhea-predominant subtype (IBS-D), constipation-predominant subtype (IBS-C), and IBS with mixed bowel habits, i.e., diarrhea and constipation (IBS-M). Growing scientific evidence supports that IBS is a disorder of brain-gut interactions that is classified by GI symptoms related to any combination of the following: motility disturbance, visceral hypersensitivity, altered mucosal and immune function, altered gut microbiota, and altered central nervous system (CNS) processing. While our understanding of IBS has increased, it is still not completely understood. This has contributed to the lack of a diagnostic biomarker. Many patients with IBS, particularly those with moderate to severe symptoms, feel that there is a lack of satisfactory treatment despite the availability of diet, over-the-counter remedies, prescription medications and behavioral therapies.
Chronic Constipation has a prevalence of 14% and occurs more commonly in women. Symptoms include decreased stool frequency (<3 bowel movements per week), hard stools, straining, sensation of incomplete evacuation, use of manual maneuvers to evacuate stool and sensation of anorectal blockage. There are three main subtypes of chronic constipation: normal transit constipation, slow transit constipation and defecatory disorders. Unlike the other two subtypes, the pathophysiology of normal transit constipation has been poorly studied and patients are generally dissatisfied with treatment. In normal transit constipation, abdominal symptoms including discomfort, bloating and sensation of incomplete evacuation are often substantial. While normal transit constipation is thought to overlap with constipation-predominant IBS (IBS-C), they don’t meet criteria for IBS due to the lack of predominant abdominal pain associated with constipation. However, more recently chronic constipation and IBS-C are thought to exist on a spectrum. Similar to IBS, diet, exercise, over-the-counter remedies, and prescription medications are used to treat constipation.
An extensive preclinical and clinical literature supports a model for IBS which is based on altered bidirectional interactions between gut and brain, with a modulatory role of the gut microbiota. Increased perception of visceral (and other sensory) stimuli, comorbidity with other chronic disorders characterized by hyperarousal, and altered activity of the autonomic nervous system are key components of this model, in addition to a number of reported peripheral abnormalities. The validity of the brain gut model of IBS has been supported by the clinical effectiveness of several mind targeted therapies, in particular gut directed hypnosis, cognitive behavioral therapy (CBT) and mindfulness-based stress reduction. In some of these studies, the clinical response was accompanied by distinct brain change.
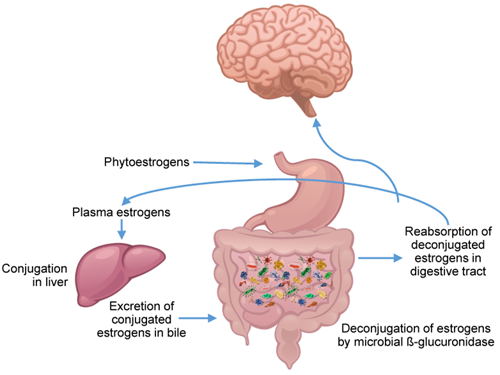 The role of estrogens in modulation of the BGM axis
The role of estrogens in modulation of the BGM axisEstrogens play a key role in maintaining homeostasis in healthy premenopausal women, and they modulate the BGM interactions at several levels of the central nervous system (CNS), including brainstem nuclei and key regions of the emotional arousal network. The UCLA SCORE proposal is built on the general hypothesis that these estrogen-BGM interactions are in part responsible for sex-related clinical features of IBS, both in terms of increased prevalence and severity in women, multisensory sensitivity, and in symptom variations associated with sex hormonal fluctuations in women. Identifying the role of estrogen-mediated nervous system modulation is a key aim of this proposal.
Evidence supports a bi-directional relationship between plasma estrogen levels and the gut microbiome. Gut microbiota can metabolize dietary estrogen-like compounds (phytoestrogens) into biologically active forms, and estrogen-like compounds may promote the proliferation and growth of certain types of bacteria. Phytoestrogens and bile acid-secreted conjugated estrogens are deconjugated by the gut microbiome through bacterial secretion of β-glucuronidase. The metabolized estrogen and phytoestrogens are then reabsorbed by the gut, and translocate into the bloodstream and can reach multiple sites, including the brain where they can bind to different types of estrogen receptors on brainstem nuclei, as well as regions within different brain networks. Microbial diversity is strongly positively associated with increased estrogen metabolite concentrations in plasma compared to parent estrogens present in the stool. At the same time, a reduction in gut microbiota diversity leads to a decrease in. estrogen metabolism and in systemic estrogen levels through a lack of estrogen metabolizing bacteria and other metabolic effects (including a reduction of short chain fatty acids (SCFAs). In postmenopausal women, the reduction in gut microbiota diversity reduces the estrabolome (i.e., intestinal microbiota genes that are capable of producing enzymes that metabolize estrogens) and therefore a reduced ability of the microbiome to deconjugate fecal estrogens, which in turn can lead to lower levels of systemic estrogen, and higher IBS symptoms. In other words, such menopause-related and gut microbiome-mediated decrease in systemic estrogen levels may contribute to the postmenopausal increase in IBS symptoms.
Based on our preliminary results, we hypothesize that nuclei in the dorsal pons can be influenced by gut microbial metabolites acting on spinal and vagal afferents in the gut, or via the systemic circulation, or both. Preclinical studies support the importance of vagal afferents in transmitting microbial signals from the gut to limbic brain networks. Given the close connections between the vagal nucleus tractus solitarius with locus coeruleus (LC) and dorsal raphe nucleus (DRN), these nuclei may serve as a relay in distributing microbial information from the gut lumen to the brain. On the other hand, metabolites (like kynurenine and certain estrogen metabolites that are absorbed and can pass the blood brain barrier) can reach the CNS through the bloodstream.
CBT is an effective treatment for IBS and is hypothesized to positively impact the brain networks related to salience assessment (reduction of biased predictions), heightened emotional arousal and negative affect associated with IBS and other chronic pain disorders. Although clinical responses in IBS suggest this may be the case, little research of these mechanisms in the context of CBT treatment has been performed. Our preliminary studies, examining both brain and microbiome data from a subsample of the recently completed IBS CBT study provides evidence that the CBT response is a) predicted by relative abundances of gut microbiota, b) associated by changes in these relative abundances, and c) associated with a significant reduction in connectivity between brainstem and relevant brain networks, and between emotional arousal, salience and sensorimotor networks, consistent with another report in the literature.
 Significance of SABV in IBS Research and Treatment
Significance of SABV in IBS Research and TreatmentThe current lack of effective and long-lasting treatments for IBS and other disorders of brain gut interactions can be attributed to various factors, including lack of progress in understanding the complexity of BGM interactions, continued reliance on symptom- based diagnostic criteria and sub classifications, lack of attention to SABV in IBS research, continued exclusive focus on gut-directed pharmacological therapies, and the until recently longstanding skepticism of the field towards the effectiveness of mind-targeted therapies. The major barriers to progress towards a comprehensive understanding of sex differences and female-specific sex hormone variations in BGM interactions in IBS include:
- Failure to consider SABV in the mechanisms and treatment outcomes in IBS. A large number of reported preclinical studies on mechanisms and treatment outcomes were performed in male animals, while clinical studies have generally enrolled insufficient number of male patients to identify possible sex-related differences. The majority of mechanistic studies in humans have not taken SABV into account.
- Failure to incorporate detailed characterization of brainstem mechanisms in IBS, despite strong preclinical evidence. While a considerable body of preclinical evidence exists about the role of the pontine LC and the noradrenergic arousal system during stress, studies on alterations in the structure, function and connectivity of brainstem mechanism in IBS patients has been limited to several studies published by the UCLA SCORE. However, these earlier imaging studies did not have sufficient spatial resolution to clearly identify the specific brainstem structures involved and did not use the advanced analyses techniques available today.
- Lack of efforts to study SABV in IBS associated alterations in the gut microbiome and in BGM interaction. No clinical studies have addressed the possible role of SABV in the relative abundance of gut microbiota, in their function or in the role of these sex related microbiome differences on IBS symptoms. Similarly, there is no published evidence of female-specific hormonal variations in brainstem nuclei and their connections to cortical networks, or in the outcomes of cognitive behavioral therapy (CBT) trials.
- Lack of information about the role of SABV in treatment-related changes in BGM interactions. While the effectiveness of mind and brain targeted therapies, in particular CBT, has been established in well-controlled clinical studies, the role of SABV in the treatment responses is not known. This is in part be explained by the difficulties recruiting sufficient numbers of men with IBS for clinical studies, and in part by a failure to take known sex differences into consideration.
