The role of brain-gut microbiome interactions in mediating IBS and constipation symptoms during menses and menopause
Overall Goal
To identify brain-gut microbiome (BGM) alterations in women with irritable bowel syndrome (IBS) and normal transit constipation. We specifically aim to evaluate BGM alterations in low estrogen states (i.e., late-luteal phase of menstrual cycle, post-menopause) and determine if these explain the increased symptom severity demonstrated during these states.
Background
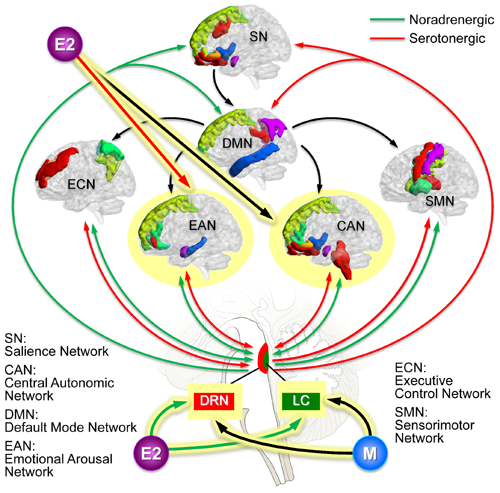
Estradiol (E2) also mediates an increase in norepinephrine (NE) signaling to the brain which increases arousal and is thought to contribute to the greater prevalence of disorders characterized by hyperarousal, including IBS in premenopausal women.
Animal studies have demonstrated estrogen receptors on regions within the dorsal raphe nucleus (DRN) and that estradiol increases central serotonin (5-HT) in the brain. However, estrogen is thought to also enhance corticolimbic inhibitory pathways (e.g., reduce amygdala responsiveness and increase hippocampal activity) that reduce emotional arousal and salience networks. We hypothesize that low estrogen states such as menopause and premenses, are associated with a reduction both in ascending arousal signals and a simultaneous reduction in central inhibitory effects on emotional arousal networks, which outweighs the reduced effects of the ascending pathways from the brainstem. If this hypothesis is correct, it could explain the increase in IBS symptoms.
General Hypotheses
- IBS symptom severity in female IBS increases during times of low or declining estrogen states, namely in menopause and mid to late-luteal phase of the menstrual cycle, and is due to reduced estrogen-dependent corticolimbic inhibition of emotional arousal networks, resulting in enhanced visceral perception. Alterations in circulating gut microbial metabolites, in particular tryptophan (TRP), estrogen, and bile acids affecting brain stem nuclei may play an additional role.
- The increased prevalence and severity of normal transit constipation in women (in the absence of changes in transit or defecation), is due to altered perception of normal, non-noxious afferent signals from the colon resulting from altered central processing. This is due to widespread changes in sensorimotor, salience, and emotional arousal networks and is in part related to increased input from ascending arousal systems originating in brainstem nuclei. Changes in the activity of ascending projections from these brainstem nuclei may be related in part to the influence of gut microbial metabolites and of estrogen.
Specific Aims
We will test these hypotheses in two specific aims in which we will measure the abundance of gut microbial taxa, total microbial gene content, plasma and gut microbial metabolites related to estrogen and TRP, and multimodal brain MRI in women subjects:
Aim A. Compare GI symptoms and brain-gut microbiome (BGM) interactions in premenopausal and postmenopausal female IBS and healthy controls (HCs)
We will compare stool microbial composition and metabolites and multimodal brain MRI (functional resting state connectivity and pain threat paradigm) in pre- and postmenopausal female IBS and HCs and in premenopausal female IBS and HCs during the follicular and mid-luteal menstrual cycle phases.
Aim B. Compare BGM interactions in premenopausal women with normal transit constipation with slow transit constipation and HCs.
We will compare stool microbial composition, metabolites and multimodal brain MRI (functional resting state connectivity and pain threat paradigm), as in Aim A, in women with normal transit constipation, slow transit constipation and HCs.
People
Project Leads: Lin Chang, MD (Co-Lead) and Arpana Gupta, PhD (Co-Lead)

Lin Chang, MD
Director, Functional GI Disroders Program, UCLA G. Oppenheimer Center for Neurobiology of Stress and Resilience; Vice-Chief, Vatche and Tamar Manoukian Division of Digestive Diseases, David Geffen School of Medicine at UCLA
Lin Chang is a gastroenterologist and physician scientist who serves as Co-Director of the G. Oppenheimer Center for Neurobiology of Stress and Resilience, an interdisciplinary center with a research and clinical focus on the interactions of pain, stress and emotions in health and disease. She has served as Co-Director of the Administrative Core of our Center’s NIH Specialized Centers of Research (SCOR), which has been funded for the past 16 years. Our SCOR has focused on sex differences in brain-gut interactions mainly with regard to irritable bowel syndrome (IBS). She has served as PI of one of the projects for each cycle. For this SCORE renewal, she will serve as Multi-PI, Co-Lead of Project 1, Co-Lead of the Career Enhancement Core (CEC), and Co-Lead of the Administrative Core. As Co-Lead of the CEC, she will oversee the collaboration with the pilot and feasibility programs to provide seed grant funding, oversee the recruitment and mentoring of young investigators, and organization of educational conferences. She has been performing clinical and translational research studies, including clinical treatment trials for 25 years. Her research has focused on brain-gut interactions, specifically pathophysiologic mechanisms, clinical symptoms, health outcomes, and treatment in IBS. She has mentored 3 gastroenterology research fellows on the UCLA Gastroenterology T32 training grant in addition to 10 clinical GI fellows, 11 medical residents, 3 post-docs, 8 visiting scientists, 10 medical students, and 5 pre-med students. Her leadership positions include Vice-Chief of the Division of Digestive Diseases at UCLA, Program Director of the UCLA GI Fellowship Program, Clinical Research Councilor of the AGA Governing Board, President of the American Neurogastroenterology and Motility Society (ANMS), member of the Rome Foundation Board of Directors. She currently serving a 4-year term on the NIH Clinical, Integrative and Molecular Gastroenterology Study Section and FDA GI Advisory Committee.
Publications
http://www.ncbi.nlm.nih.gov/sites/myncbi/lin.chang.1/bibliography/40548865/public

Arpana Gupta, PhD
Co-Director, Neuroimaging and Bioinformatics Core, G. Oppenheimer Center for Neurobiology of Stress and Resilience; Assistant Professor, Vatche and Tamar Manoukian Division of Digestive Diseases, David Geffen School of Medicine at UCLA
Arpana Gupta is an Assistant Professor and Director of the Neuroimaging Core at the UCLA G. Oppenheimer Center of Neurobiology of Stress and Resilience (CNSR); where she specializes in research that investigates the interactions between environmental and biological factors in shaping neurobiological phenotypes associated with stress-based diseases such as obesity and metabolic syndrome. Her current program of research, broadly defined, is based on developing a model that aims to understand the bidirectional interaction of the brain with those in the periphery (immune cells, gut microbiota-related metabolites), and the modification of these interactions by vulnerability or protective factors (adverse life events, sex, race, socioeconomic status [SES], resilience, diet) related to obesity and ingestive behaviors. More recently she has been investigating diet interventions in altering the brain-gut microbiome axis on health and disease. Another main area of interest is sex differences in central responses related to the brain-gut microbiome axis, as well as its relationship to various disease states. She applies advanced multivariate analytic techniques in order to integrate data from multiple neuroimaging sources, inflammatory markers, microbiome and metabolite profiles, and behavioral data, in order to determine the unique variance associated with altered brain gut microbiome axis in specific disorders. In 2016, she received a mentored K23 grant and in 2020 a R03 grant from the NIH NIDDK to investigate the brain-gut microbiome influences in obesity. She has also received funding from the AGA Rome Foundation, Biocodex, and pilot funds from the UCLA CURE/CTSI program.
Publications
http://www.ncbi.nlm.nih.gov/sites/myncbi/1v_1kl872tPQf/bibliography/46222127/public
Studies
Coming soon
