Sex Differences in Fatty Acid and Mitochondrial Metabolism are Associated with Diastolic Dysfunction in Heart Failure
Yang Cao1, Laurent Vergnes2,3, Calvin Pan1, Yuchen Wang1, Karthickeyan Chella Krishnan1, Timothy M. Moore1, Zhiqiang Zhou1, Sarada Charugundla1, Christoph Rau4, Marcus M. Seldin1, Jessica Wang1, Yibin Wang4, Karen Reue2,3,5, and Aldons J. Lusis1,2,6
1Division of Cardiology, Department of Medicine, University of California, Los Angeles, CA, USA; 2Metabolism Theme, David Geffen School of Medicine at UCLA, Los Angeles, CA, USA; 3Department of Human Genetics, David Geffen School of Medicine at UCLA, Los Angeles, CA, USA; 4Division of Molecular Medicine, Departments of Anesthesiology, Medicine, and Physiology, Molecular Biology Institute, Cardiovascular Research Laboratories, David Geffen School of Medicine at University of California, Los Angeles; 5Molecular Biology Institute at UCLA, Los Angeles, CA, USA; 6Department of Microbiology, Immunology and Molecular Genetics, University of California, Los Angeles, CA, USA
Heart failure with preserved ejection fraction (HFpEF) is an increasingly prevalent syndrome with diastolic dysfunction and preserved ejection fraction. Women are about twice as likely to develop HFpEF as compared to men and they present with a greater symptom burden, but the factors underlying the sex differences are unknown. Here we examine the genetics of diastolic dysfunction by applying HFpEF model to a panel of diverse inbred strains of mice known as Hybrid Mouse Diversity Panel (HMDP). We found a strong genetic component showing complex inheritance and, as in humans, female mice exhibited higher E/A ratio and E/e’ ratio in the progression of HFpEF, suggesting that females are more susceptible to diastolic dysfunction (Figure 1A-1C). Gonadectomized mice showed an increase in E/e’ ratio, demonstrating that sex hormones affect diastolic performance (Figure 1D). Compared with male control mice, female mice and gonadectomized mice exhibited a decrease in mitochondrial function and fatty acid metabolism upon the development of HFpEF (Figure 1E-1G). Consistently, female hearts exhibited reduced mtDNA copy number as compared to males (Figure 1H). Gonadectomy in males reduced mtDNA content, whereas it increased mtDNA in females (Figure 1I), demonstrating that mitochondrial function exhibits sexually dimorphic regulation. Further, cardiac mtDNA was inversely associated with cardiac traits such as lung mass and mitral inflow E velocity (Figure 1J), suggesting that mitochondrial function contributes to sex differences in HFpEF. We further identified sex-specific regulators of diastolic function by integrating HF/HS HMDP with HFpEF cohort as well as human ischemic heart failure cohort. At the intersection of these three cohorts was Acsl6, an enzyme involved in fatty acid metabolism (Figure 2A). Acsl6 expression was lower in female hearts vs. males and was further decreased after HFpEF induction (Fig. 2B-2C), suggesting its contribution to increased mitochondrial and metabolic dysfunction in female HFpEF mice. In addition, expression quantitative trait loci (eQTL) analysis of Acsl6 revealed that Acsl6 was regulated locally (cis-eQTL, peak SNP rs26974045) and genetic variation in Acsl6 expression was significantly correlated with key traits of diastolic function including E/A ratio, mtDNA copy number and body weight (Figure 2D). Importantly, Acsl6 overexpression in female heart by AAV administration was sufficient to decrease heart weight/tibia length, E/e’ ratio, glucose intolerance and plasma unesterified cholesterol in HFpEF mice (Fig. 2E-2J), demonstrating that Acsl6 overexpression attenuates diastolic dysfunction, reduces metabolic disorder and maintains metabolic homeostasis in the progression of HFpEF. Acls6 provides a clue for the design of early-phase clinical trials using agents that specifically target mitochondria and metabolism to improve symptoms in patients with HFpEF.
In conclusion, our findings suggest that sex-specific differences in mitochondrial function and fatty acid metabolism underlie, in part, the diastolic dysfunction associated with HFpEF. They also favor the development of sex-specific strategies from the prevention, detection, and therapeutic management of HFpEF.
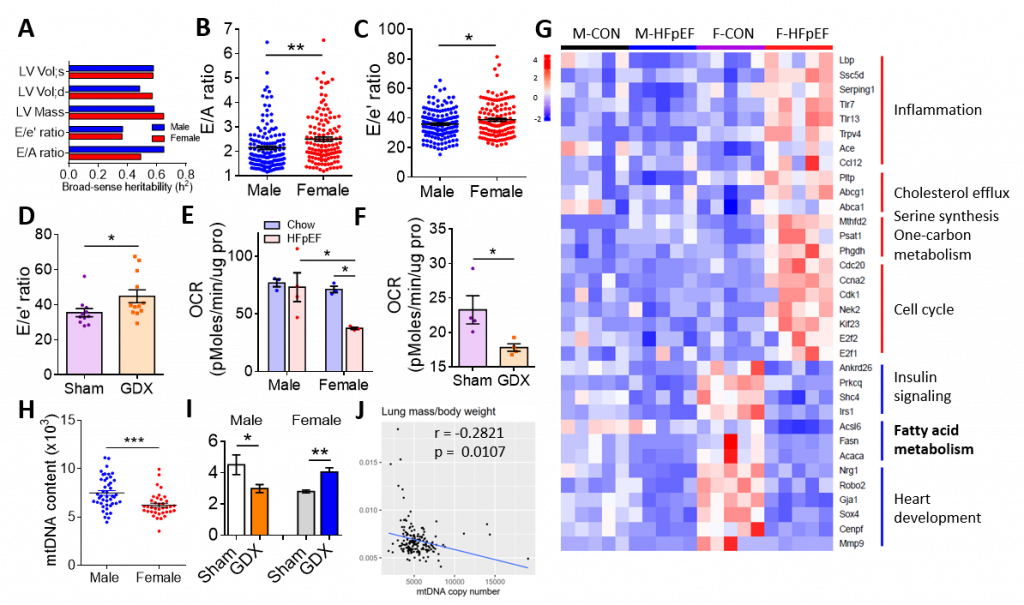 |
Figure 1. Worse diastolic function in female mice was associated with a decrease in fatty acid metabolism and mitochondrial function. A-C. Heritability of cardiac parameters (A), E/A (B) and E/e’ ratio (C) of 30 HMDP strains fed with HFD + l-NAME (two-hit) for 5 weeks. D. E/e’ ratio of C57BL/6J male mice after gonadectomy (GDX) and 5 weeks of HFD + l-NAME diet. E. Oxygen consumption rates (OCR) of isolated mitochondria from C57BL/6J male and female hearts were measured by Seahorse assay. F. OCR was measured in C57BL/6J male mice after GDX followed by 5 weeks of HFD + l-NAME feeding. Substrate is palmitoyl-carnitine. G. Heatmap showing relative gene expression and related pathways in heart tissue of male (M) and female (F) mice after 5 weeks of chow diet (CON) and HFD + l-NAME diet (HFpEF). H. mtDNA content in the heart of 30 strains of male and female mice after 5 weeks of HFD + l-NAME diet. I. mtDNA content in the heart of male and female mice after GDX. J. mtDNA content inversely correlated with lung mass in HMDP mice treated with 3 weeks of isoproterenol (ISO). *p< 0.05, **p<0.01, ***p<0.001. Each point represents a mouse. |
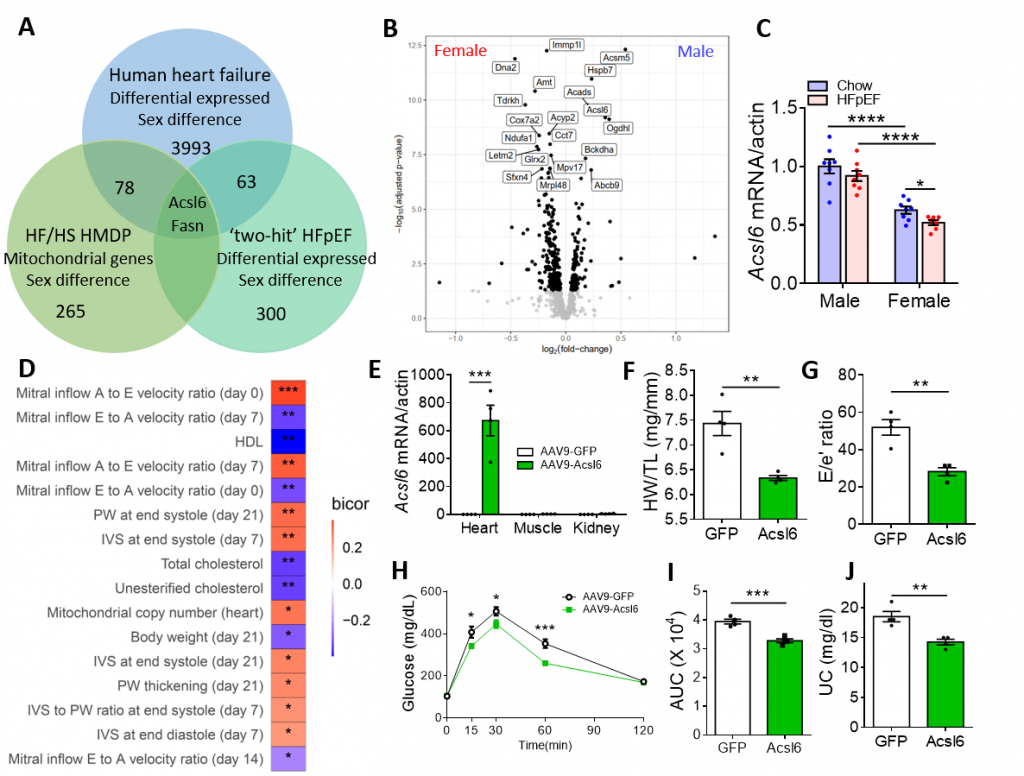 |
Figure 2. Acsl6 is a cis-regulator of diastolic function. A. Venn diagram showing the overlap of sex-specific mitochondrial genes in human and mouse cohorts: high fat/high sucrose (HF/HS)-induced metabolic syndrome HMDP cohort, HFpEF cohort and recently published human ischemic heart failure cohort. B. Volcano plot showing mitochondrial differential expression genes in male vs. female heart tissue after 8 weeks of HF/HS feeding. C. qRT-PCR showing mRNA levels of Acsl6 (Acyl-CoA Synthetase Long Chain Family Member 6) in male and female hearts under chow and HFpEF condition. D. The correlation between local genetic variation in Acsl6 expression and parameters of diastolic function and metabolism in ISO-HMDP. E. qRT-PCR showing overexpression of Acsl6 by AAV9 in the heart but not in other tissue. F-J. HW/TL ratio (F), E/e’ ratio (G), Glucose tolerance test (H), area under curve of GTT (I) and plasma unesterified cholesterol (J) of C57BL/6J female mice treated with AAV9 and 5 weeks of HFD + l-NAME feeding. *p< 0.05, **p<0.01, ***p<0.001. Each point represents a mouse. |
Breakout Room: Cao, Yang
View Poster: https://uclacns.org/symposium2021/3-Cao-Yang.pdf
