Epigenetic Changes Associated with Sex and Their Role in Irritable Bowel Syndrome Pathophysiology
Swapna Mahurkar-Joshi1-3, Elizabeth J Videlock2,3, Charalabos Pothoulakis2,3, Emeran A Mayer1-3, Lin Chang1-3
1G. Oppenheimer Center for Neurobiology of Stress and Resilience, 2Vatche and Tamar Manoukian Division of Digestive Diseases, 3David Geffen School of Medicine at UCLA
Background: IBS is a female-predominant, stress-sensitive disorder characterized by abdominal pain, altered bowel habits and increased early adverse life events (EALs). Studies suggest that EALs can lead to long-lasting epigenetic changes in stress-related genes. DNA methylation changes associated with sex have been reported, however, their role in peripheral pathophysiologic mechanisms in IBS is not clear. Aims: To identify 1) differentially methylated genes in the colonic mucosa of IBS patients compared to healthy controls (HCs), 2) potential DNA methylation-based subtypes within IBS, 3) sex-specific IBS-associated DNA methylation changes, and 4) changes in expression of differentially methylated genes associated with IBS and sex. Methods: Sigmoid colon biopsies from 102 IBS patients (66% [N=66] F; 36 IBS-Diarrhea, 36 IBS-Constipation, 30 IBS-Mixed) and 36 age matched HCs (55%[N=20]) were examined. Gastrointestinal (GI) symptoms were measured using validated questionnaires. DNA methylation at >450,000 CpG sites was measured using HM450 array and gene expression from biopsies was measured using 3’QuantSeq RNAseq. Data were analyzed using ‘minfi’ package in R. DNA methylation levels of autosomal CpG sites were compared between IBS and HCs in men and women combined and separately using linear model for arrays. Gene Ontology (GO) was assessed using DAVID annotation tools. FDR<0.05 or p<0.001 was considered significant. Results: Overall women vs men: 3662 autosomal CpG sites in the colonic mucosa were significantly differentially methylated in men vs women (FDR<0.05). Overall IBS vs HCs and methylation-based IBS subtypes: Between IBS and HCs, 209 sites were different (p<0.001). Within IBS, consensus clustering of 5000 most variant CpGs revealed 3 methylation-based clusters. A methylation signature of 2964 CpG sites (FDR<0.05, mean difference >3%) differentiated divergent clusters (Cluster 1 vs 3, Fig 1A). Cluster 1, which included more men compared to cluster 3 (p=0.017) was associated with higher abdominal pain and overall symptom severity (Fig 1B) and enriched in ion channel and neurotransmitter transport genes (FDR<0.05). Interaction between IBS and sex: Within women, more CpGs were differentially methylated in IBS vs HCs compared to within men (401 vs 230 CpGs, p<0.001) with very little overlap (1%). Increased hyper-methylation in promoter regions was seen in IBS men, while increased gene body methylation was seen in IBS women. Combined DNA methylation and gene expression signature discriminated IBS men from HC men better than IBS and HCs within women (Fig 2). In women, the IBS-associated differential methylation of genes was related to cell adhesion and neuronal function (p<0.05), whereas in men, the differential methylation in IBS vs HCs was related to immune pathways. Conclusions: DNA methylation changes in the colonic mucosa were present in IBS vs HCs. and associated with symptom severity. Epigenetic changes affecting genes involved in immune related pathways were seen in IBS men, while DNA methylation changes were seen in cell adhesion and neuronal pathways in IBS women. Further studies are needed to delineate these mechanisms.
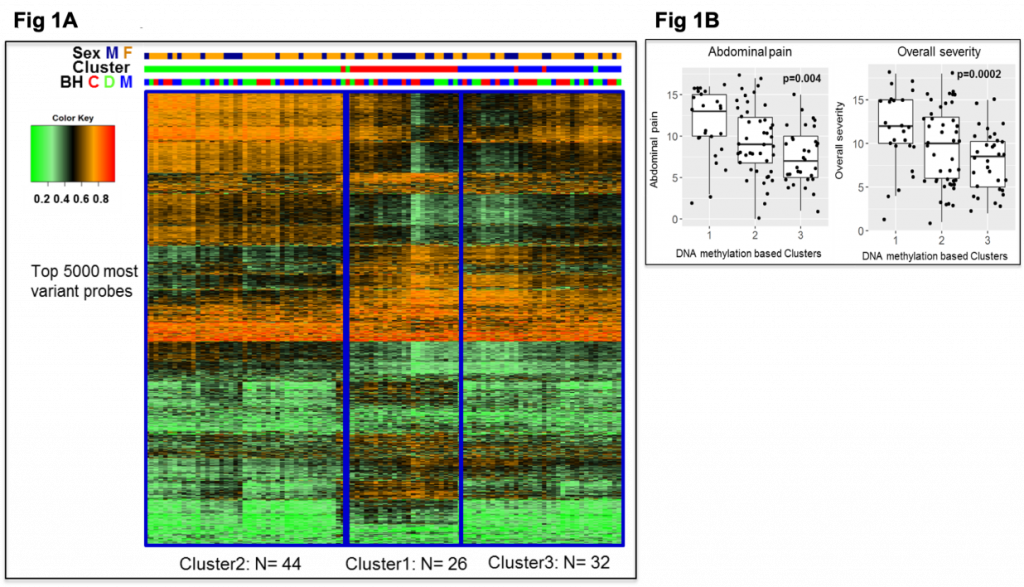 |
Fig 1: DNA methylation-based subgroups in colon and their association with pain and severity in IBS
Legend: Fig 1A: Heatmap colors range from green (unmethylated) to red (methylated); the variable bars represent (from bottom) 1. Bowel habits (BH): red- IBS-C (C), green-IBS-D (D), blue-IBS-M (M); 2. Cluster: Cluster2: Green; Cluster1: red; Cluster3: Blue; 3. Sex: Male: blue; Female: orange. |
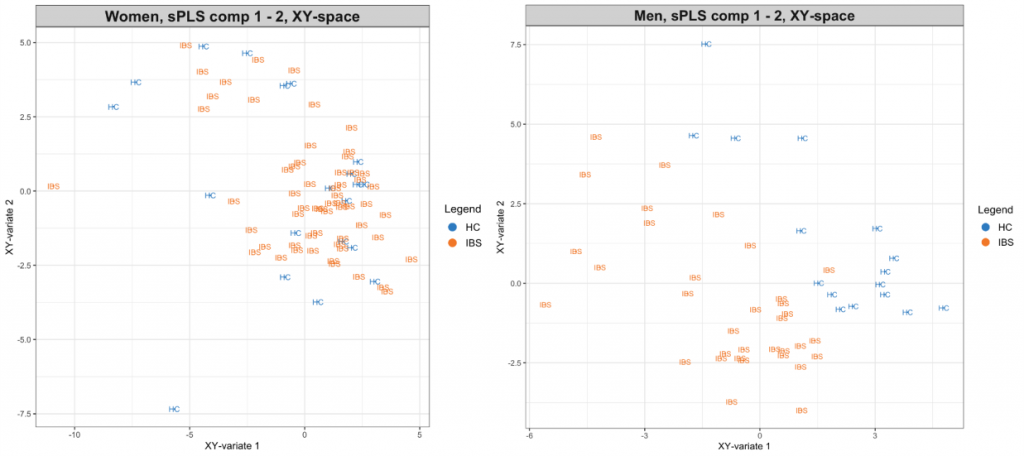 |
Fig 2: Combined gene expression and methylation profile of IBS-associated genes in women and men. Legend: XY-space, DNA methylation and gene expression combined PCA components |
Breakout Room: Joshi, Swapna
View Poster: https://uclacns.org/symposium2021/13-Joshi-Swapna.pdf
